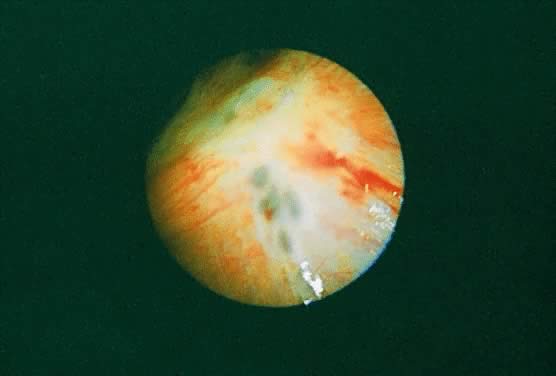
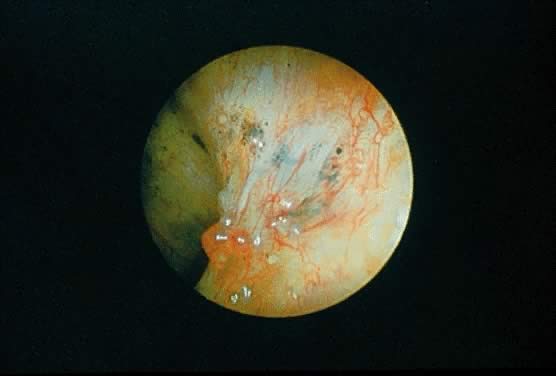
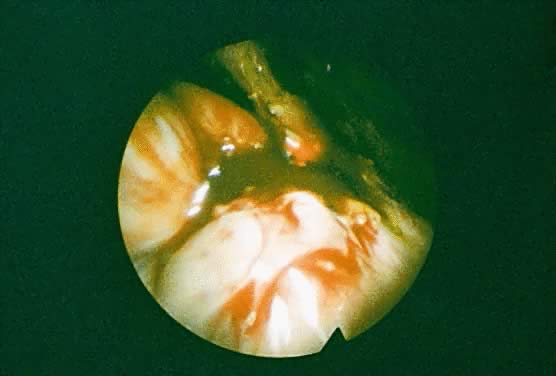
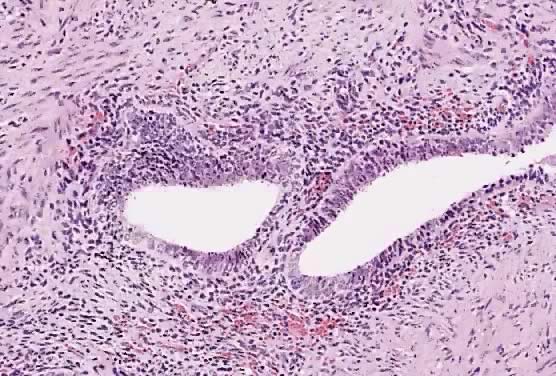
In the treatment of endometriosis, diverse therapeutic approaches, including
no treatment, medical treatment, surgical treatment, and a combination
of medical and surgical treatment, have been used to address the
clinical consequences (i.e. pain, infertility, and pelvic masses). The use of analgesia (e.g. nonsteroidal antiinflammatory drugs) may also be considered in patients
with pain. Despite shortcomings in the classification system on which
the studies evaluating treatment outcome are based, sound clinical decisions
regarding treatment must be made with the available data. Initial
assessment of the reproductive goals of the patient is essential
before initiating treatment. Moreover, a clear understanding of the treatment
options and the desired end point can facilitate the formulation
of a treatment plan to address the individual needs of each patient. Evaluation of Outcome Data From the perspective of the patient, outcomes assessment data are the most
relevant. The effectiveness of a particular treatment in reducing
pain or increasing the monthly chance of conceiving is the question addressed
by numerous clinical studies. Prospective, randomized, controlled
trials are required to determine the most effective treatment approach. Unfortunately, most
studies are uncontrolled or retrospective, making
them prone to selection bias. As a result, these studies are more
likely to conclude that a treatment is efficacious when in actuality
it is not. In evaluating the clinical response to infertility treatment, consideration
should be given to the method of data reporting and analysis. Crude
or simple pregnancy rates are simple to calculate and have been commonly
used. Because pregnancy rates increase with longer patient follow-up, crude
pregnancy rates are of limited value. Life-table analysis
or fecundity rates have been used to account for the time-dependent nature
of pregnancy. Although these methods are preferred for use in studies
investigating the effectiveness of infertility treatment, they cannot
correct for selection bias. Meta-analysis is another useful tool
because the data from several studies are pooled together. This method
of analysis, however, does not correct for the different lengths of follow-up
among the studies. Moreover, the subtleties of the data from
each study may be lost in the process. Another consideration in infertility studies is the background conception
rate. In many situations, endometriosis is associated with a relative
decrease in fertility rather than absolute infertility. Pregnancies
occur at a background rate in these women without any intervention. This
rate must be determined to assess the efficacy of a particular treatment. In assessing therapeutic interventions for endometriosis-associated pain, difficulties
are encountered in the objective assessment of the outcome
measure of pain because of its subjective and heterogeneous nature. The
perception of pain is the manifestation of a complex interaction
of physiologic processes, sensory thresholds, personality, culture, mood, psychologic
influences, and knowledge regarding pain. Another important
factor is the proper accounting for the significant placebo effect
associated with pain treatment. A partial response to placebo treatment
has been reported in up to 55% of patients with endometriosis-associated
pain.173 For treatments that are effective in relieving pain, a recurrence of symptoms
can be expected in a number of patients. Investigation of the
recurrence rate is important for a more complete evaluation of a particular
therapeutic regimen. Although it is difficult to make definitive
conclusions regarding the efficacy of therapeutic interventions, knowledge
of the data can assist in making a more rational decision regarding
treatment. Medical Treatment Medical treatment consists of hormonal therapy that most commonly includes
combination oral contraceptives, high-dose progestins, danazol, and
GnRH agonists. The basis of pharmacologic therapy is that endometriosis
implants are capable of responding to hormones. These medications
interrupt the cycle of stimulation and bleeding of ectopic endometrial
tissue and induce atrophy of the implants, decreasing pain and the inflammatory
response that may cause fibrosis and adhesions. Unfortunately, current
medical therapy is not definitive in the treatment of endometriosis
because fibrosis and adhesions cannot be removed and recurrence
of endometriosis may occur after cessation of treatment. In the treatment of infertility, medical therapy alone or in combination
with surgery does not improve pregnancy rates in women with endometriosis-associated
infertility.174 Medical therapy results in additional costs, side effects, and requisite
contraception period, rendering this approach inadvisable in patients
whose only symptom is infertility. The combination of medical therapy
with surgery, such as the preoperative use of GnRH agonists for severe
endometriosis, may be implemented as indicated to control pelvic pain
before a delayed surgery or possibly to improve the pelvis before
surgery.175 Preoperative medical treatment, however, has not been shown to improve
postoperative pregnancy rates. In contrast, medical treatment for pain is effective in reducing symptoms.176,177 Uncontrolled trials have shown an improvement in pain symptoms in approximately 80% to 90% of
patients receiving highdose progestin, danazol, gestrinone, or
GnRH agonists.178–181 Estimates of recurrence rates vary with a positive correlation between
the probability of recurrence and severity of disease. A recurrence rate
up to approximately 50% per year has been reported.182 As with any medical treatment, the benefits of pain relief must be weighed
against the likely occurrence of undesirable side effects that may
limit the use of a medication, potentially rendering it less effective. ORAL CONTRACEPTIVES. The goal of estrogen and progestins administered as oral contraceptives
is to induce a pseudopregnancy state. These hormones promote decidualization
and eventually atrophy of endometrial implants with continued
treatment, interfering with the growth of endometriosis.183,184 Another potential benefit of oral contraceptive use includes reduction
of retrograde menstrual flow from the resulting amenorrhea associated
with continuous daily use. Because of the initial proliferative response
of endometrial implants to treatment, an exacerbation of symptoms
may be encountered in the first 2 to 3 months before improvement is seen. As
a result, treatment is usually continued for at least 6 to 12 months. The
more common side effects are nausea, weight gain, fluid retention, breast
tenderness, mood changes, and breakthrough bleeding. No
prospective, controlled studies have shown that treatment with oral contraceptives
results in resolution of disease or improved pregnancy rates. Many
clinicians, however, feel that they are a reasonable, relatively
inexpensive, and effective method to control endometriosis. PROGESTINS. Progestins are another often considered medication for the treatment of
endometriosis that acts in a similar fashion to oral contraceptives
by promoting decidualization and atrophy of endometrial tissue. The most
commonly used progestin for endometriosis is the progesterone derivative
medroxyprogesterone acetate (MPA). Depot MPA, progesterone-derived
megestrol acetate, and the 19-nortestosterone derivative norethindrone
also have been used. Although menstruation is usually suppressed with
these medications, breakthrough bleeding is a common problem, occurring
in 38% to 47% of patients.185 Other common side effects, which are reversible, include nausea, breast
tenderness, fluid retention, and depression. The typical oral dose of MPA is 30 mg daily for 3 to 6 months. The use
of MPA at this dose and megestrol acetate appear to provide significant
pain relief.179,186 In a prospective, randomized, placebo-controlled study, high-dose MPA (100 mg/day) for 6 months
followed by a 6-month observation period resulted
in pain relief comparable to danazol and significantly better than
placebo.176 Moreover, after this 1-year period, laparoscopy revealed a significant
reduction in disease volume compared with placebo, which was as effective
as danazol. A reduction in high-density-lipoprotein cholesterol levels
with high-dose MPA has been reported.187 The significance of this reduction, however, is unknown. In contrast to its ability to relieve pain, progestins are not effective
in improving endometriosis-associated infertility. In a nonrandomized, controlled
study of women with early-stage endometriosis, no difference
in the cumulative pregnancy rates at 30 months was observed with
oral MPA, danazol, or expectant management.188 Similarly, in a study of women at all stages of endometriosis treated
with high-dose MPA, danazol, or placebo, the pregnancy rates among the
treatment groups were not different after 30 months of observation. Moreover, progestins
suppress ovulation, delaying the opportunity for conception. Progestin
therapy is generally not recommended for patients
with endometriosis-associated infertility. DANAZOL. Danazol is an orally active isoxazol derivative of 17α-ethinyl testosterone (ethisterone) that has been used for endometriosis treatment
since 1971.189 The clinical effects of hypoestrogenic vaginal changes, vasomotor symptoms, and
endometrial atrophy associated with ovarian inactivity from
danazol use have resulted in the description of a “pseudomenopause.” Danazol, however, does not alter basal levels of gonadotropins. Suppression
of the midcycle follicle-stimulating hormone and luteinizing
hormone surge occurs, resulting in a chronic anovulatory state
and amenorrhea.190,191 Many other hormonal effects have been attributed to danazol. Danazol inhibits
multiple enzymes in the steroidogenesis pathway192 and increases free testosterone levels by displacing it from sex hormone-binding
globulin (SHBG).193 Estradiol is also displaced from SHBG along with progesterone and cortisol
from corticosteroid-binding globulin. Danazol binds well to the androgen
receptor194 and interacts with progestational and glucocorticoid cytosol receptors.195,196 Danazol is associated with numerous side effects197 (Table 2) that are primarily attributable to its androgenic activity and have limited
its widespread use among patients. Although most of the side effects
are reversible, some may be irreversible such as deepening of the
voice,198 and the occurrence of this side effect is an indication for discontinuing
the medication. The potential metabolic side effects include increased
serum enzyme levels possibly associated with cholestatic jaundice,199,200 decreased SHBG levels,201 and decreased levels of high-density lipoproteins.187,202 These alterations are expected to return to normal after completion of
therapy within several weeks. Long-term use of danazol has raised some
concerns regarding the risk of atherosclerotic heart disease due to
changes in the lipid profile. TABLE 2. Side Effects of Danazol Treatment
Side Effects | Incidence (%) |
Weight gain* | 85 |
Muscle cramps | 52 |
Decreased breast size | 48 |
Flushing | 42 |
Mood change | 38 |
Oiliness, skin or hair | 37 |
Depression | 32 |
Sweating | 32 |
Edema | 28 |
Change in appetite | 28 |
Acne | 27 |
Fatigue | 25 |
Hirsutism | 21 |
Decreased libido | 20 |
Nausea | 17 |
Headache | 17 |
Dizziness | 10 |
Insomnia | 10 |
Rash | 8 |
Increased libido | 8 |
Deepening of voice | 7 |
*0–1 lb, 15%; 1–5 lb, 22%; 6–10 lb, 32%; 11–15 lb, 18%; 16–20 lb, 11%.
Adapted from Buttram VC et al: Treatment of endometriosis with danazol: report
of a 6-year prospective study. Fertil Steril 37:478, 1982.
The recommended dosage of danazol has varied, with most authorities recommending 400 to 800 mg
daily in divided doses. The duration of treatment
is usually 6 months. The general consensus is that amenorrhea must
be induced for danazol to be effective. At 600 to 800 mg/day, danazol
produced amenorrhea in 90% to 100% of women.203 The 400 and 200 mg/day doses resulted in amenorrhea in 80% and 44% of
women, respectively. To minimize side effects, danazol is occasionally
started at 400 mg/day and titrated upward as tolerated until the desired
effect is achieved. Symptomatic improvement in pain has been reported with danazol treatment
in many trials. After 6 months of danazol, improvement in pain was observed
in up to 90% of women.204 In a randomized, controlled study, pain reduction was significantly better
in women treated with danazol compared with placebo for up to 6 months
after discontinuation of therapy.176 In the same study, a reduction in apparent implants was noted in 60% of
danazol-treated women compared with 18% in the placebo group. Unfortunately, danazol
along with the other pharmacologic interventions are
unable to reduce the amount of adhesions and the pain that may result
from them. The long-term recurrence of pain has not been well studied. One
report, however, suggests that the pain symptom recurrence rate is
about 50% per year.205 The effectiveness of danazol in treating infertility has been less encouraging. One
randomized, prospective study of danazol treatment of minimal
endometriosis with 12 months of follow-up resulted in a cumulative
pregnancy rate of 37.2% in the danazol group and 57.4% in the untreated
group.204 In another randomized, placebo-controlled study of all stages of endometriosis
with 30 months of follow-up, the cumulative pregnancy rates in
the danazol group and placebo group were 33% and 46%, respectively.206 In light of these studies and the inability to treat anatomic distortions, danazol
has no proven benefit in the treatment of endometriosis-associated
infertility. GESTRINONE. Gestrinone is a 19-nortestosterone derivative that acts as an androgen
receptor agonist and a progesterone receptor agonist or antagonist. Treatment
with this steroid results in amenorrhea and endometrial atrophy
similar to danazol.207,208 The potential side effects, which are similar to those of danazol, tend
to be mild and transient. Compared with danazol, the incidence of side
effects is less with gestrinone.209 Moreover, no adverse effects on the lipid profile or liver function tests
were noted. Because of the long half-life, gestrinone is typically administered in
oral doses two or three times weekly (5 to 10 mg/week). After 6 months
of treatment, a significant reduction in endometriosis implants was observed
in a randomized, placebocontrolled study.210 Gestrinone also appears to be effective in reducing pain symptoms.210 In the treatment of infertility, however, gestrinone did not improve the
cumulative pregnancy rate compared with placebo or a control group
with unexplained infertility.211 There is no evidence to support the use of gestrinone for infertility
treatment in patients with endometriosis. GONADOTROPIN-RELEASING HORMONE ANALOGS. The GnRH analogs are synthetic decapeptides that have substitutions at
the 6 and sometimes at the 10 position relative to the naturally occurring
GnRH decapeptide. These synthetic analogs are long-acting and downregulate
the pituitary gland, resulting in a reversible decrease in
gonadotropins and subsequent “medical oophorectomy” with continued
use.212 The amount of time required to reach castrate levels of estradiol is approximately 3 to 6 weeks. Various forms of GnRH analogs are available
that can be administered intranasally, by injection, or as a subcutaneous
pellet. All of the available analogs are effective in producing hypoestrogenism
and amenorrhea. The potential side effects of GnRH analogs include hot flashes, vaginal
dryness, irregular vaginal bleeding, insomnia, depression, fatigue, irritability, headache, and
decreased libido. Compared with danazol, the
analogs produced more hot flashes and vaginal dryness but less weight
gain, mood changes, and fluid retention.177 One of the primary concerns with the prolonged use of GnRH analogs is
the potential for bone demineralization because of hypoestrogenism.213 The trabecular bone loss appears to be reversible if therapy is limited
to 6 months.214 The gonadal suppression obtained with GnRH analogs results in endometriosis
implant regression and pain relief. The GnRH analogs were as effective
as danazol in causing regression of endometriosis implants.177,182,215 Similarly, pain relief also has been demonstrated after treatment. After
leuprolide acetate treatment, a decrease in pelvic pain, dysmenorrhea, pelvic
tenderness, and pelvic induration was observed compared with
placebo.216 GnRH analogs also compare favorably to danazol in treating pain symptoms.215,217,218 The recurrence rate of pain after completion of treatment is less clear. One
study reported about one half the patients experiencing recurrence
of symptoms by 1 year.182 Treatment of endometriosis-associated infertility with GnRH analogs does
not appear to be beneficial. Compared with danazol, no difference in
the pregnancy rates was observed at 12 months or 18 months of follow-up.177,182 In most situations, the use of GnRH analogs with infertility as the only
indication is generally not advisable. The adverse effect of prolonged hypoestrogenism has limited the long-term
use of GnRH analogs for the treatment of endometriosis. Because of
the recurrence rate of endometriosis after discontinuation of therapy, prolonging
treatment with these effective medications would be advantageous. To
this end, add-back therapy combined with GnRH analogs has been
employed to minimize the short- and long-term side effects of hypoestrogenism
while maintaining therapeutic efficacy.219 The various agents and regimens that have been used have generally yielded
good results.220 These regimens include progestin only (e.g. medroxyprogesterone acetate, norethindrone, norethindrone acetate, tibolone), progestin (e.g. norethindrone) plus bisphosphonate (e.g. sodium etidronate), and estrogen (e.g. conjugated equine estrogens, 17β-estradiol) plus progestin (e.g. medroxyprogesterone acetate, norethindrone). Suppression of vasomotor
and vaginal symptoms, elimination of significant bone mineral density
loss, and a decrease in pelvic pain was observed with all the add-back
regimens. Adverse alterations in the high-density- and low-density-lipoprotein
cholesterol ratio occurred with higher norethindrone doses (10 mg/day).221 Current recommendations are not to use add-back therapy for anticipated
GnRH agonist treatment periods of less than 3 months, to consider add-back
for 3- to 6-month treatment periods, and to use add-back for prolonged
GnRH agonist treatment beyond 6 months.220 The simplest add-back regimen is norethindrone acetate (5 mg/day). Other
regimens also found to be successful in alleviating symptoms and achieving
pain relief after 1 year are low-dose norethindrone (2.5 mg/day) plus
a bisphosphonate or norethindrone acetate (5 mg/day) in conjunction
with conjugated equine estrogens (0.625 mg/day). Supplemental calcium (1000 mg/day) should
be administered during treatment. Periodic
assessment of bone mineral density and lipid profiles is also recommended. Surgical Treatment The surgical approach to endometriosis may be accomplished by laparotomy
or laparoscopy. With improvements in equipment and operative technique, laparoscopy
is the most common choice for surgery. The degree of success, however, depends
on the laparoscopic skills of the surgeon. Laparoscopy
provides better visualization, less tissue trauma and exposure
to foreign bodies, possibly less adhesion formation, and lower complication
rates.222,223 Laparoscopic incisions are smaller and less painful, allowing faster recovery
times.224 The disadvantages of laparoscopy compared with laparotomy include the
lack of three-dimensional perspective, greater likelihood of operator
fatigue, inability to palpate structures, and need for expensive equipment. Laparotomy
is usually reserved for extensive enterolysis, bowel
resection, or other situations deemed too complex for the laparoscopic
approach.225 Laparoscopic elimination of endometrial implants may be accomplished by
laser ablation, electrosurgical desiccation, or sharp resection. The
type of laser used during laparoscopic surgery depends on the desired
laser characteristics for the particular clinical situation. The carbon
dioxide (CO2) laser has excellent precision (depth of tissue destruction is 0.1 mm) but
poor coagulating ability that allows the surgeon to create tissue
injury at a precise and identifiable localized area. The potassium-titanyl-phosphate
laser (KTP532) and argon lasers are less precise than
the CO2 laser but have better coagulating properties. The neodymium-doped yttrium-aluminum-garnet (Nd:YAG) laser has good coagulating properties but
poor precision (depth of tissue destruction is 4 mm) and can cause large
volume thermal injury that is invisible to the operator. Sharp resection
is effective in removing disease with a low risk of inadequate
treatment but is prone to increased bleeding. Unipolar electrosurgery
is also effective but has the risk of deeper tissue damage. Bipolar electrosurgery
may be used to desiccate endometriosis lesions. The precise
extent of tissue destruction, however, cannot be determined and carries
the risk of inadequate treatment. The techniques used during surgery are directed toward removing all endometrial
implants in an atraumatic, hemostatic fashion in the least amount
of time. The choice of instruments must reflect the best judgment
and skill level of the surgeon as to the optimal means of accomplishing
these goals. Adhesions are excised rather than simply lysed because
of the possible presence of endometriosis within adhesions. Reduction
of tissue desiccation and maintenance of a clean surgical field is facilitated
by copious irrigation with physiologic fluids. The operative
success rate correlates with meticulous surgical technique that maintains
serosal integrity and decreases the risk of de novo adhesion formation. Close adherence to surgical principles can increase
the likelihood of a successful outcome226 (Table 3). TABLE 3. Surgical Principles in the Treatment of Endometriosis
Knowledge of disease and treatment modalities
Experienced surgeon
Adequate facilities, personnel, and equipment
Appropriate patient selection
Informed consent
Proper patient position
Careful pelvic evaluation
Maximum exposure
Use of magnification
Minimum tissue trauma
Excellent hemostasis
Removal of all diseased tissue
Avoidance of foreign body material
Confirmation of tissue pathology
From Adamson GD: Laparoscopic treatment of endometriosis. In Adamson GD, Martin
DC (eds): Endoscopic Management of Gynecologic Disease. Philadelphia: Lippincott-Raven, 1996.
INFERTILITY. Surgery is usually the treatment of choice for endometriosis-associated
infertility. The advantage of surgical therapy in the treatment of infertility
is the opportunity to remove adhesions and restore normal anatomy. In
contrast to medical treatment, a period of contraception is
not required. This provides the older infertility patients a time savings
of up to 6 months, during which time fertility may decrease. In general, within 1 to 2 years after surgical therapy for endometriosis, a
pregnancy rate of approximately 65% can be expected. In a study of women
in whom endometriosis was the only known cause of infertility, about
one half of the women conceived within the first 6 months after surgery, and 86% conceived
within 15 months.227 Recurrence of endometriosis implants after surgery was reported for 28% of
patients within 18 months228 and 40.3% after 5 years.229 In a meta-analysis of studies comparing surgery with nonsurgical treatments
for all stages of endometriosis-associated infertility, the surgical
approach was found to be superior.230 In patients with minimal or mild endometriosis, laparoscopic treatment
has been used frequently because treatment can be accomplished easily
during diagnostic laparoscopy. However, the ablation or removal of endometriosis
implants potentially can increase the risk for postsurgical
adhesion formation. The decision to treat minimal or mild endometriosis
has been based on the nature and location of the lesions, on the potential
of the disease to become more advanced, and on the presence of
pain symptoms. In light of the difficulties in evaluating the data in
the literature, the lack of rigorous clinical studies showing an improvement
in fertility, and the variable length of follow-up in infertility
studies, the conventional wisdom has been that surgical treatment
for minimal or mild endometriosis does not confer an advantage over expectant
management. Some data support the surgical approach to infertile patients with minimal
or mild endometriosis. In a prospective, multicenter, double-blind, controlled, randomized
study, surgical treatment by laparoscopy resulted
in a significantly higher pregnancy rate after 36 weeks than expectant
management.231 Cautery, laser, or a combination of the two were used to treat the endometriosis
implants and adhesions. The cumulative probability of pregnancy
in the surgically treated versus nontreated group was 30.7% and 17.7%, respectively. The
corresponding fecundity rates per 100 person-months
were 4.7 and 2.4. This study provides convincing evidence that surgery
is beneficial in the treatment of minimal or mild endometriosis-associated
infertility. In the presence of moderate or severe endometriosis, surgery is the treatment
of choice for endometriosis-associated infertility. Because of
the usual anatomic distortion associated with moderate or severe endometriosis, surgery
has been the usual treatment approach. As a result, few
data exist regarding no treatment or medical treatment. The available
evidence supports the surgical approach compared with the nonsurgical
approach for invasive, adhesive, or endometriotic disease.230 Intuitively, surgical treatment that can potentially correct anatomic
defects should result in better outcomes than expectant management or
medical treatment, which does not restore normal anatomy. Because of the ineffectiveness of medical therapy, surgery has been the
approach for treating endometriomas. Complete resection of the cyst wall
is preferred to minimize thermal injury to the ovary, have greater
assurance of complete removal, and obtain a specimen for pathologic examination. In
two retrospective studies, pregnancy rates after laparoscopic
treatment of endometriomas in infertility patients were 50% (26 of 52) and 52% (12 of 23).232,233 In a prospective cohort study comparing endometrioma treatment by laparoscopy
or laparotomy, the estimated cumulative pregnancy rate at 3 years
was 52% with laparoscopy and 46% with laparotomy.234 The size or number of endometriomas did not affect the pregnancy rates. The
recurrence rate of resected endometriomas is less than 10%, with
an associated 20% incidence of de novo adhesion formation and approximately 80% incidence of recurring complete
or partial dense adhesions.232,235 After drainage and cystectomy, normal ovarian function appears to be retained.236 The posterior cul-de-sac and rectovaginal septum are locations where deeply
invading endometriosis can be found. These areas also can be the
most difficult to dissect. In one study of infertile patients with partial
or complete cul-de-sac obliteration, 74% (34 of 46) of the patients
conceived after laparoscopic treatment with 38% (13 of 34) requiring
more than one laparoscopy.237 PELVIC PAIN. Surgical treatment for endometriosis-associated pelvic pain has been poorly
studied but appears to be useful. Uncontrolled trials report success
in relieving pain in 70% to 100% of patients.238 In one study, complete relief of pain 1 year after surgery was reported
in 82% of patients.239 In a prospective, randomized, double-blind, controlled study comparing
CO2 and KTP532 laser laparoscopy treatment of pelvic pain associated with
all stages of endometriosis, an improvement or resolution of symptoms 6 months
after surgery was observed in 62.5% of treated patients and 22.6% of
untreated patients.240 Patients with higher-stage disease had more pain relief 6 months after
surgery than patients with mild disease. Another consideration in determining
the success of treatment in addition to the percentage of patients
experiencing pain relief and the duration of pain relief is the
recurrence rate of pain. Unfortunately, a return of symptoms is experienced
by at least 10% to 20% of patients treated for endometriosis-associated
pain per year.241 Deep lesions, including those that invade into the rectovaginal septum, appear
to correlate with the severity of pelvic pain.242,243 Complete excision of the lesions offers the best opportunity for relief
of pain. In one study of 250 women, surgical excision resulted in a 70% cure
rate for pelvic pain and a recurrence rate of less than 5% after 5 years.244 After 1 year, the recurrence rate for pain has been reported to be as
high as 32% after surgical excision of infiltrating rectovaginal endometriosis, with
only 2 of 151 experiencing severe pain.245 In another study, no dysmenorrhea or dyspareunia was reported after 40 months
after surgical excision of rectovaginal lesions.246 Ovarian endometriomas have been treated by various surgical techniques.247 Drainage, cyst stripping or ablation, and wedge excision had comparable
results in improving pain symptoms. After surgery, pain relief has been
observed in 61% to 100% of patients.233,247,248 The approach of wide excision and drainage has been recommended as an
alternative to wedge excision because of less adhesion formation and a
recurrence rate (23%)249 similar to that for cyst stripping and ablation. Other studies, however, have
reported an even lower recurrence rate (<5%) of endometriomas
using the stripping or ablation techniques, which are perhaps the most
widely used techniques.248,249 Definitive surgery consisting of hysterectomy and salpingo-oophorectomy
is effective for relieving endometriosis-associated pain. This approach
should be considered for patients who fail medical or conservative
surgical treatment and can accept loss of fertility. Alternatively, women
who have completed childbearing and desire a more definitive approach
to their symptoms may elect to proceed with this treatment as the
primary option. Persistence of symptoms due to adhesions, residual peritoneal
lesions, or ovarian remnant syndrome is possible during the course
of endometriosis treatment. Pain relief after hysterectomy and salpingo-oophorectomy
is observed in up to 90% of patients.15 In selected patients with no significant ovarian involvement, hysterectomy
alone is an option associated with a disease recurrence rate of approximately 7%, requiring
reoperation.250 In moderate or severe endometriosis, the risk of recurring pelvic pain
has been suggested to be as much as five times greater if the ovaries
are not removed at the time of hysterectomy. The relation between pelvic adhesions and pain is unclear. Adhesions can
distort normal anatomic relationships, potentially restricting the mobility
and distensibility of organs and causing pain.251 However, a correlation between the extent or location of adhesions and
the severity and duration of pain has yet to be demonstrated.252–254 In uncontrolled studies, adhesiolysis resulted in an improvement in pelvic
pain symptoms in approximately 85% of patients.251,255–257 Adhesion reformation was observed in 97.1% of patients and at 66% of the
sites of original adhesiolysis.258 Despite the uncertainty of the correlation between adhesions and pain, lysis
of adhesions appears reasonable in the presence of endometriosis-associated
pain, especially when the location of adhesions and pain
correlate. Presacral neurectomy involves interrupting the sympathetic innervation
to the uterus at the level of the superior hypogastric plexus. This technique
has had variable results when used to treat endometriosis-associated
pain. In a randomized, prospective study, presacral neurectomy
effectively relieved dysmenorrhea but had variable effects on dyspareunia, lateral
pain, and back pain.259 In another randomized, controlled study, however, no significant difference
in the frequency and severity of dysmenorrhea, pelvic pain, and
dyspareunia was observed.260 Because of the variable results, only selected patients with primarily
midline dysmenorrhea unresponsive to conservative treatment should be
considered for presacral neurectomy. Laparoscopic uterosacral nerve ablation (LUNA) is another procedure used
for the treatment of endometriosis-associated pain. In a prospective, randomized, double-blind
study of women with intractable dysmenorrhea, pain
relief after LUNA was observed in 81% of women after 3 months, compared
with no pain relief in all the women not having LUNA.261 However, the recurrence of pain after 1 year occurred in approximately
one half of the women. For minimal to moderate endometriosis, another
study showed improvement in pain symptoms after LUNA and ablation of
endometriosis in 62% of women, compared with 23% of untreated women.240 It appears reasonable to perform a LUNA when the uterosacral ligaments
are involved with endometriosis or when patients with central chronic
pain are unresponsive to other therapies. Other Treatment Infertility associated with endometriosis may be addressed by advanced
reproductive technologies. A variety of treatment options can be offered, depending
on the clinical situation. After an infertility evaluation, the
most common treatments are controlled ovarian hyperstimulation
with or without intrauterine insemination and the assisted reproductive
technologies (ART). These treatments are intended to increase the overall
fecundity and do not directly cause regression of endometriosis
implants. Overall, clomiphene citrate treatment does not appear to be better than
no treatment, whereas gonadotropin treatment appears to at least double
the monthly fecundity. In a controlled, randomized study of women with
minimal or mild endometriosis, controlled ovarian hyperstimulation
with gonadotropins resulted in a monthly fecundity rate of 15%, compared
with 4.5% in untreated women.262 Similar conclusions were reached in another study of women with minimal
or mild endometriosis, demonstrating the superiority of gonadotropin
and intrauterine insemination over no treatment.263 The addition of intrauterine insemination to clomiphene citrate and gonadotropin
therapy appears to be helpful in increasing pregnancy rates.264 Although data on the treatment of moderate or severe endometriosis are
relatively sparse, the expectation is for no improvement in pregnancy
rates with ovulation induction because of the higher probability of significant
anatomic distortion that would interfere with oocyte transport
to the fallopian tube. The assisted reproductive technologies refer to procedures such as in vitro fertilization (IVF), gamete intrafallopian transfer (GIFT), zygote intra-fallopian
transfer (ZIFT), tubal embryo transfer (TET), intracytoplasmic
sperm injection (ICSI), and assisted hatching. When assessing outcome
according to diagnosis, patients with endometriosis were reported
to have lower pregnancy rates compared with patients with tubal factor
infertility.265,266 Based on the observation of lower pregnancy rates from oocytes donors
with endometriosis and no decrease in pregnancy rates in oocyte recipients
with endometriosis, poor oocyte quality was thought to be responsible
for the adverse effect on the pregnancy rate in patients with endometriosis.265 Other studies, however, found no difference in IVF success rates between
patients with and without endometriosis.267,268 Similarly, conflicting data regarding the success rates according to stage
of endometriosis have been reported.266–268 It is difficult to make definitive conclusions regarding the effects of
endometriosis on success rates. In light of the potential adverse effects of moderate or severe endometriosis
on the oocyte, it seems reasonable to consider surgical treatment
of moderate or severe disease before initiating IVF cycles. In a prospective, randomized
study, however, no difference in pregnancy rates
or live birth rates was observed between patients who were treated or
not treated for endometriosis at the time of GIFT.269 No definitive conclusions can be made regarding surgical treatment of
endometriosis before ART. Medical suppression with GnRH agonists for 2 to 3 months
may also be considered.270 Frequently, surgery has been performed earlier during the course of the
infertility workup. IVF is usually considered after expectant management
or controlled ovarian hyperstimulation with intrauterine inseminations
has failed after surgery. |